To pig farmer Thomas Titus, new scientific techniques could bring better disease resistance for his herd, saving baby pigs and potentially millions of dollars for the pork industry.
That is the foremost benefit “when I think about the possibilities of CRISPR (also called CRISPR-Cas9, the leading gene editing technique) and how it could help my farm,” says Titus, who raises corn, soybeans and pigs near Elkhart, Ill.

Thomas Titus farms near Elkhart, Illinois
The devastating PRRS virus causes disease in two ways: a respiratory form that weakens young pigs' ability to breathe and a more severe reproductive form that causes pigs to die during late pregnancy. In North America alone, PRRS is estimated to costs producers $600 million annually.
“As a pig farmer, I see that one of the greatest diseases that impacts every pig farm across the United States is PRRS (porcine reproductive and respiratory syndrome). If there’s an opportunity for us to eradicate diseases like that – or have resistance to diseases like that – it would be just astronomical!”
 Indeed, at least two companies say they can deliver just what Titus says he wants. Both Genus, which owns a patent to its technique, and Acceligen, a division of Recombinetics, also involved in developing the trait, report they can infuse pigs with PRRS resistance.
Indeed, at least two companies say they can deliver just what Titus says he wants. Both Genus, which owns a patent to its technique, and Acceligen, a division of Recombinetics, also involved in developing the trait, report they can infuse pigs with PRRS resistance.
The Genus procedure, for example, deletes a pig gene that the virus needs as a doorway to the animal’s cells. But, as with other gene editing (GE) discoveries, those PRRS remedies can’t happen until the U.S. government approves these new animals.
More broadly, researchers are eager for the U.S. and other governments to decide how to use science and risk-based criteria to regulate this new approach to genetic engineering (the topic of the next article in this series). That’s why commercialization is still a few years out.
For now, the Food and Drug Administration (FDA) defines all intentional DNA alterations in animals as drugs, irrespective of how or why they were changed.
“It’s a nonsensical position,” says Alison Van Eenennaam, a Cooperative Extension Specialist in Animal Genomics and Biotechnology at the University of California-Davis.
“If I didn’t know any better, I would think the (FDA) regulations were written by Greenpeace because they are not science-based,” she emphasized during a recent speech at the American Farm Bureau Federation. “We can’t run all of these animals through a drug evaluation.”
But while the regulatory issues are being sorted out, researchers and industry leaders are marching ahead with these new scientific tools, along with ongoing efforts to use current animal-improving practices like artificial insemination, embryo transfer and genetic selection.
Van Eenennaam says there’s an “urgent need to ensure the science-based framework focused on these novel products is adopted. Or else it’s going to block U.S. ranchers from having access to this technology.”
“We’re very excited by the potential for gene editing, and not only against PRRS … a devastating disease to the industry,” said veterinarian Dan Kovich, speaking for the National Pork Producers Council. “In the future,” he said, “looking to other applications for disease resistance, prevention, management – all sorts of traits -- I think the potential is there (for gene editing) traits that can have an impact on animal welfare, reducing the need for antibiotics . . .”

Alison Van Eenennaam, a Cooperative Extension Specialist in Animal Genomics and Biotechnology at the University of California - Davis
“This is very different from the GMOs (genetically modified organisms) that people have talked about in the past,” Kovich says, noting that in gene editing, no genes from foreign species are introduced. “I think there are very sound reasons why the marketplace will be accepting of this technology. This is a very precise technology, working within the genome of the pig. It’s not transgenics.” So, he says, NPPC is “just really excited about where this can go.”
Swine-related gene editing discoveries are but a small part in the leading edge of genetics research. Even though the new genetic altering procedures such as CRISPR (Clustered, Regularly Interspaced, Short Palindromic Repeats) and TALEN (Transcription Activator-Like Effector Nucleases) are just a few years old, scientists have already invented some more refined versions of even those techniques – for example, modifying the performance of specific genes without actually cutting or removing any genes in the nucleus, as CRISPR and TALEN do.
Scientists around the globe are giving the genetic manipulations test drives: cutting, splicing, deleting and amplifying genes within the cells of everything from viruses and fungi up the biological scale to mammals and the human body.
One significant feature of all the gene editing work is that most of it is taking place not in the cell nuclei of higher life forms, but of the lowest.
Megan Hochstrasser, science communications manager for the Innovative Genomics Institute at UC-Davis, keeps a roster of new research postings of gene-editing products developed with CRISPR-Cas9, the most frequently used new gene-editing system.

Megan Hochstrasser, science communications manager for the Innovative Genomics Institute at the UC-Davis
“It seems that the microbes section is growing the most rapidly,” she said. So far, 88 of 208 posted CRISPR successes are about microorganisms: fungi, bacteria, and protists (molds, algae, etc.). Another 46 are on invertebrates (insects, snails, worms, etc.), 39 alter crops and other plants, and 10 are on fish.
Thus, only a handful of successful genome editing advances– just 9 percent in Hochstrasser’s count – directly alter the biological makeup of mammals or birds. But such genome amendments could also be significant to human health.
Two years ago, an immunologist in London, for example, was able to extract immunity cells, called T-cells, from the blood of a donor, modify them with a TALEN gene-editing procedure so the body of a young human recipient of the cells would not reject them. The altered T-cells were then used to replace a childhood leukemia patient’s own T-cells. The child gained full remission from leukemia.
Most of the gene editing successes have worked within the animal’s own genes, not injected ones from other species, and are intended to reduce suffering, infections, and injuries.
For example, Acceligen is working with Van Eenennaam and scientists at UC-Davis to gain government approval for its genetic amendment for polled cattle, which means that they don’t grow horns. A few breeds are naturally hornless. Cattle with horns can injure others in the herd or people handling the cattle. Meanwhile, dehorning adds to management chores for ranchers and means pain and blood loss for calves.
Tad Sonstegard, Acceligen’s chief scientific officer, explained that isolating precise gene amendments for the polled trait can allow ranchers to make that sole change directly to a herd. Making a single targeted change can relieve the breeder from needing several generations to rebuild the genetic profile of the herd.
Note that Australians found that while European-origin cattle (including American breeds) assimilate Acceligen’s polled trait, hot-climate cattle such as Brahman and Bos indicus (zebu) cattle raised in northern Australia, do not. So Australian researchers did their own gene editing to select the polled trait for their breeds, allowing the rancher to breed cattle with double-dominant genes for the “true-polled” inherited hornless trait.
Another pain-reduction application of gene editing on tap in the swine sector is eliminating the need for surgical castration to produce favorable tasting pork. For swine, castration is done to avoid so-called boar taint, an unpleasant odor and taste in pork.
Recombinetics and DNA Genetics, a swine genetics supplier, have joined ranks to perfect a breeding technology that results in male piglets remaining in a pre-puberty state – thus, naturally castrated, in effect. Besides avoiding pain for piglets, GE castration would save labor and avoid risk of infection from the surgical procedure.
“We are trying to make and male and female parents both sterile, and they will only be able to reproduce if someone administers spawning hormones to them. So then the resulting offspring will all be sterile,” Dunham said. He notes that some fish breeds are naturally that way, and won’t breed unless certain hormones are present at high enough levels in the water.

Rex Dunham, Auburn University
In the aquaculture sector, meanwhile, Auburn University geneticist Rex Dunham has inactivated three genes in catfish cell nuclei, which direct release of reproductive hormones that allow the fish to breed.
“The primary purpose,” he explains, “is to have complete reproductive control over transgenic or other types of gene-edited fish. So we could almost entirely eliminate any possibility of environmental impact, and completely prevent them from interbreeding (with other catfish).”
His gene editing appears successful so far, Dunham reports. In his first parent generation, “we have really strong evidence that these fish were sterile or had greatly reduced reproductive capacity,” he said, noting that complete adoption is never achieved in the first generation.
Significantly, most of animal-related gene editing to date doesn’t mess directly with the genomes of humans or higher life forms. Nearly all of it involves modifying simpler life forms to improve health for people, farm animals and wildlife, and most of it, like the PRRS remedy, targets diseases or harmful pests.
Gene editing, in fact, portends even more direct benefits to human health. Possible herpes resistance, for example. Nearly all adults are infected with types of herpes viruses, a group that cause cold sores, keratitis, genital herpes, shingles and mononucleosis. The viruses can remain dormant for long periods and then spring up. But scientists now suspect that they can use CRISPR genome editing to inactivate herpes by attacking its genes directly within people’s virus-infected cells.
Meanwhile, gene editing is sure to spell more human medical blessings via farm animals, often called farmaceuticals.
A few such advances, using transgenic procedures, have already been approved, because they offer clear benefits for people. In 2006, for example, the biotech-reluctant European Union (and in 2009, the U.S.) green-lighted a breed of goat modified to produce a human anti-clotting protein, called antithrombin alfa, in its milk. The protein is needed to treat people with hereditary antithrombin deficiency, a rare clotting disorder. Now, it can be harvested from the goat’s blood, instead of from a human donor’s blood, the traditional source. Also, the EU and U.S. have both approved a genetically-modified chicken that lays eggs containing an anti-cholesterol drug.
 Kris Huson, communications manager for Recombinetics, explains that her company, like many other biomedical research entities, likes to employ pigs as surrogates for developing health remedies for people.
Kris Huson, communications manager for Recombinetics, explains that her company, like many other biomedical research entities, likes to employ pigs as surrogates for developing health remedies for people.
“Now, particularly with gene editing – and pigs share so much DNA with humans and human diseases – they are just a superior animal model,” she said.
Her company has modified pig genomes so the animals can develop colon cancer, human heart disease, Alzheimer’s, diabetes, and other human disorders and/or resistance to such disorders, giving researchers targets to pursue human health remedies.
Mice have long been used as a model for human health research, she points out. “But it is a big stretch to go from a mouse heart to a human heart,” she said, so pigs, whose organ sizes are similar to human ones, have become highly desired for clinical research.
“The next step is using pigs as an ‘oincubator,’ we are calling it, -- sort of an incubator -- to grow human tissue cells and organs in the context of a pig.” She asks: “Could we one day grow organs for patients: a cornea, lung tissue, hepatocytes, pancreas cells?”
For anyone on a transplant waiting list – and there are plenty – that’s welcome news. In the U.S. today, more than 116,000 people are waiting for an organ transplant, according to orgondonor.gov . Roughly 20 people per day die waiting.
Recombinetics is working on pig embryos that had been injected with human stem cells to begin growing organs containing human cells, creating what’s known as a chimera, scientists reported in January 2017. Chimeras have cells from two different types of organisms.
Researchers hope they will eventually be able to grow new human body parts for transplantation – a transplant organ that’s uniquely made to match a human’s DNA. But for now, they need to figure out how to get more human cells to survive inside of their pig, cow or sheep hosts.
SAB Biotherapeutics, on the other hand, uses cattle as its living factory of antibodies to help people fight disease, in part because cattle are big, thus turn out big volumes of antibodies. Its genetically designed cattle, Tc Bovine, produce a menu of antibodies the same way that other cattle or people make antibodies, except they produce human antibodies instead of cattle ones. Its antibodies can be aimed at “prevention and treatment of diseases from Ebola to cancer, and diabetes to influenza,” the company says on its website.
Chicken eggs, too, have been harnessed as factories for biomedical research and health care. Trouble is, a lot of children are allergic to eggs. So they can’t get some immunization shots, since many major vaccines are cultured in chicken eggs.
Australian researchers report, however, that they have altered an egg-white gene largely responsible for the allergy, making the egg hypoallergenic. That suggests hypoallergenic egg-based vaccines may be on the way.
The dynamics of editing animal genomes changes when the editing is done on wild species or when unlimited, worldwide adoption of such an edit is contemplated. As with the Dunham goal of raising sterile catfish, breeders think about the need for a brake pedal, so that a dominant and potentially harmful trait, doesn’t enforce itself around the world.
Intrexon, for example, developed male Oxitec mosquitos that pass on to offspring a self-limiting trait wherein the offspring don’t live to adulthood. As a result, the local mosquito population declines.
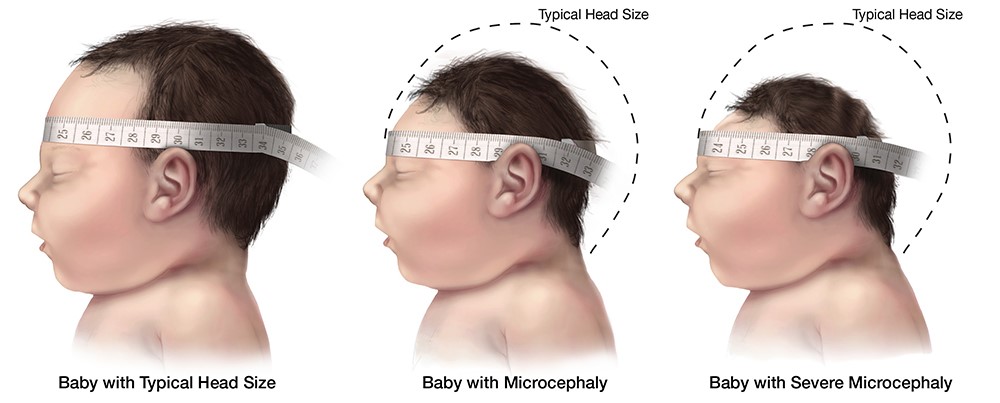
Babies with Congenital Zika Virus Infection have smaller head sizes and other abnormalities. Source: Centers for Disease Control
Those mosquitoes have been spread in recent years in Brazil, Panama, the Cayman Islands and Florida in efforts to control the mosquito population that carry Zika, dengue and other dangerous viruses. A Zika virus infection during pregnancy created alarm for thousands of women when it was identified as a cause of congenital brain abnormalities.
On the other hand, consider what may be the biggest weapon for controlling organisms in the wild – a gene drive.The genetic tweak employed by Intrexon means female mosquitoes will have dead-end offspring when mating with the mutant males; not when they mate with other males.
The concept is simple. Just ensure the gene or genes for adding or blocking a trait are on both chromosomes – thus, a double dominant modification that will be carried to practically all offspring. For example, a gene drive could single out a species, like pesky rats, to potentially eliminate.
Scientists working at the University of Edinburgh’s Roslin Institute are using CRISPR gene editing techniques as a way to spread infertility in rats and mice – similar to the technique that already works for mosquito control – so that an entire population could be eliminated within a few generations.
The gene drive has selling points, and the attraction is clear if the goal is pest control. That is, a gene drive is toxin-free, eliminates a producer’s costs and cuts down on the environmental and health risks of using pesticides. What’s more, it avoids collateral damage: unnecessary harm to insects, bacteria, fungi, rodents and other critters who are beneficial or harmless.

Spotted wing drosophila eating a strawberry. Source: Clemson University
“The promise of a gene drive, from a technical point of view, is that you can make a very small change at the genome level and release a single mosquito, a single mouse . . . and have a very large change across a whole population,” said Jim Thomas, co-executive director of ETC Group, a California based ecological policy consultant, speaking at a UC-Davis conference on gene-editing.
“Here we have an exceedingly powerful technology, and we have to be exceedingly careful,” he said.
One solution: Scientists at the University of California, Riverside, have already installed a gene drive in the drosophila that would potentially wipe out the insect over time. Its use would require regulatory approval. Siccing a gene drive on the spotted-wing drosophila, for example, holds the potential for greatly reducing billions of dollars annually in damage that pest unleashes on cherries, berries and other stone fruits in the West Coast region, in Europe and elsewhere. This Asian native fruit fly lays its eggs in ripening fruit – instead of in rotting fruit, as other fruit flies do – making the fruit unmarketable.
On an even larger scale, using a gene drive on another strain of mosquito – the top malaria-spreading one -- might save millions of people in Africa and Asia from illness and death. More than 200 million people get the parasitic disease annually, and nearly half a million die from it, according to the World Malaria Report 2017.
Years ago, UC, Irvine biologist Anthony James found specific genes in Anopheles gambiae, a types of mosquito, that pass a malaria-resistance on to offspring. Those with the trait can carry the disease but are not able to spread it. More recently, he and fellow scientists have been funded to develop a gene drive to spread that resistance to all offspring of that strain of mosquito.
Field testing a malaria mosquito gene drive will probably occur in Africa, where malaria is by far the most prevalent. But James tells Agri-Pulse that it is “hard to predict” when it will start or in which countries.
“There are significant scientific, regulatory and social challenges to be met” before breeding such mosquitoes in the open, he says. He notes that the hosting countries will have to approve the work.
What’s more, scientists at Imperial College London have prepared their own mosquito gene drive: this one to impose infertility on female offspring of the same malaria mosquitoes.
As with most biotech advances, there is fear about possible outcomes, especially, in this case, runaway unintended results in the wild.
The Broad Institute of the Massachusetts Institute of Technology and Harvard, which hold the patent for CRISPR-Cas9, discourage its use for gene drives. Also, a year ago, environmental advocates pressed world governments at their meeting as the United Nations Convention on Biodiversity, to initiate a ban on gene drives. Scientists there persuaded the gathering to reject that move, but the technology will undoubtedly face a lot of opposition worldwide.

Ryan Phelan, a wildlife conservationist and executive director of Revive & Restore
Ryan Phelan, a wildlife conservationist and executive director of Revive & Restore, agreed at the UC-Davis, CRISPR conference that the gene drive concept “does bring up huge issues” when it comes to protecting wildlife, especially threatened species. She asks: “Who gets to decide how you adopt something that knows no bounds?”
Yet, she says, in trying to protect wildlife, “there are some problems that are so intractable,” and she thinks gene editing may have to be one of the remedies pursued while mankind still has an opportunity to try solutions.
Phelan points, for instance, to an invasive strain of mosquito in Hawaii that spreads avian malaria, which has already killed off some wild species of birds there and threatens the survival of others, including the native honeycreeper. Plus the impact on the birds is worsening as climate change brings higher temperatures – and more avian malaria mosquitoes – into Hawaiian mountain habitat.
“Gene drive could indeed be part of an ultimate solution,” Phelan opines. “But gene drive technology needs more iterations before we could safely test gene drives in the wild,” and she says, and applying the technology would have to be “fully transparent and closely engage local and indigenous communities in Hawaii.”
On the other hand, she says, “avian malaria should not be in Hawaii.” Plus, the islands there have their own native mosquitos; the avian malaria one has no value in the food chain. So, she asks, “Do we have a right, or a moral obligation, as stewards, to remove those mosquitoes” via a gene drive or other biotech remedy?
It’s important to always remember that, “science and innovation always outruns law and policy. These ethical and moral questions are not new,” said Bill Even, CEO of the National Pork Board. “They arise every time a new technology emerges.”
“I would have these same discussions when I worked at Pioneer,” Even recalls about some of the Iowa-based seed company’s early research aimed at improving corn yields.
“When Henry Wallace pioneered the use of hybrid seed corn in the 1920’s, there were all sorts of people saying ‘the sky is falling,’ ‘you’re messing with God,’ and this is ‘not the natural way things should happen.’ There was all this fearmongering.
“Now, it’s viewed as one of the most successful improvements in agriculture and modern history. And people assume it’s natural and they welcome it,” Even added.
For more news, go to: www.Agri-Pulse.com



