Animal and plant breeders are trying out a set of powerful new tools which have the potential to revolutionize agricultural practices and provide consumers with more healthy and safe food options.
Their new toolbox is called gene editing, and the instruments in it have strange-looking names: endonucleases, for example, which are enzymes breeders can use to sever selected DNA proteins on an organism’s chromosomes, allowing the breeder to make changes at the DNA break points, and thus alter the organism’s genetic makeup.
 “We use the term ‘gene editing’ rather loosely” in the world of science, said Bernice Slutsky, senior vice president for the American Seed Trade Association. At its core, gene editing is “plant breeding innovation,” she said. Plant breeders have always used a range of tools – a toolbox of different disciplines.” With the new techniques, they are “doing the same things that breeders have always done, but very precisely,” she said.
“We use the term ‘gene editing’ rather loosely” in the world of science, said Bernice Slutsky, senior vice president for the American Seed Trade Association. At its core, gene editing is “plant breeding innovation,” she said. Plant breeders have always used a range of tools – a toolbox of different disciplines.” With the new techniques, they are “doing the same things that breeders have always done, but very precisely,” she said.

Bernice Slutsky, American Seed Trade Association
The precision Slutsky describes did not suddenly burst forth full-blown but is what researchers have built in recent decades. Some milestones include:
- Development of a technique called polymerase chain reaction in the 1980s. It allows researchers to duplicate a fragment of DNA proteins thousands or millions of times, providing a quick and cheap supply of specimens for their research.
- Another key gene editing tool, zinc-finger nucleases, emerged more than a decade ago. ZFN are enzymes that target sequences of genes on chromosomes where genetic amendments are sought. This is similar to the newer gene editing processes but is considered more laborious and often less successful.
- What’s more, in the past two decades or so, scientists have laid the table, so to speak, for gene editing by locating and cataloging – called sequencing – the entire genomes of a multitude of plants and animals. That makes virtually every gene potentially available for breeders to find and amend or delete. Full sequencing has been done for cattle, chickens and pigs. In addition, the sequenced genomes of almost 200 different plant species have been published, according to Todd Michael, who’s been tracking plant sequencing as director of informatics for the J. Craig Venter Institute in California.
With such advances in place, two processes developed in recent years are accelerating breeders’ ability to genetically alter crops and animals and apply the brakes to harmful organisms. Both can precisely improve a plant or animal without incorporating DNA from another species. One process is a mouthful called Clustered Regularly Interspaced Short Palindromic Repeats, or CRISPR, and the other is a similarly large swallow called Transcription Activator-Like Effector Nucleases (TALEN).
So, how do these genome-amending systems work?
Some have described CRISPR-Cas to be like editing text in a word processing application. With a specific goal in mind, the CRISPR-Cas system performs a specific search within DNA – an organism’s complete set of instructions – to delete, edit or replace target genetic sequences.
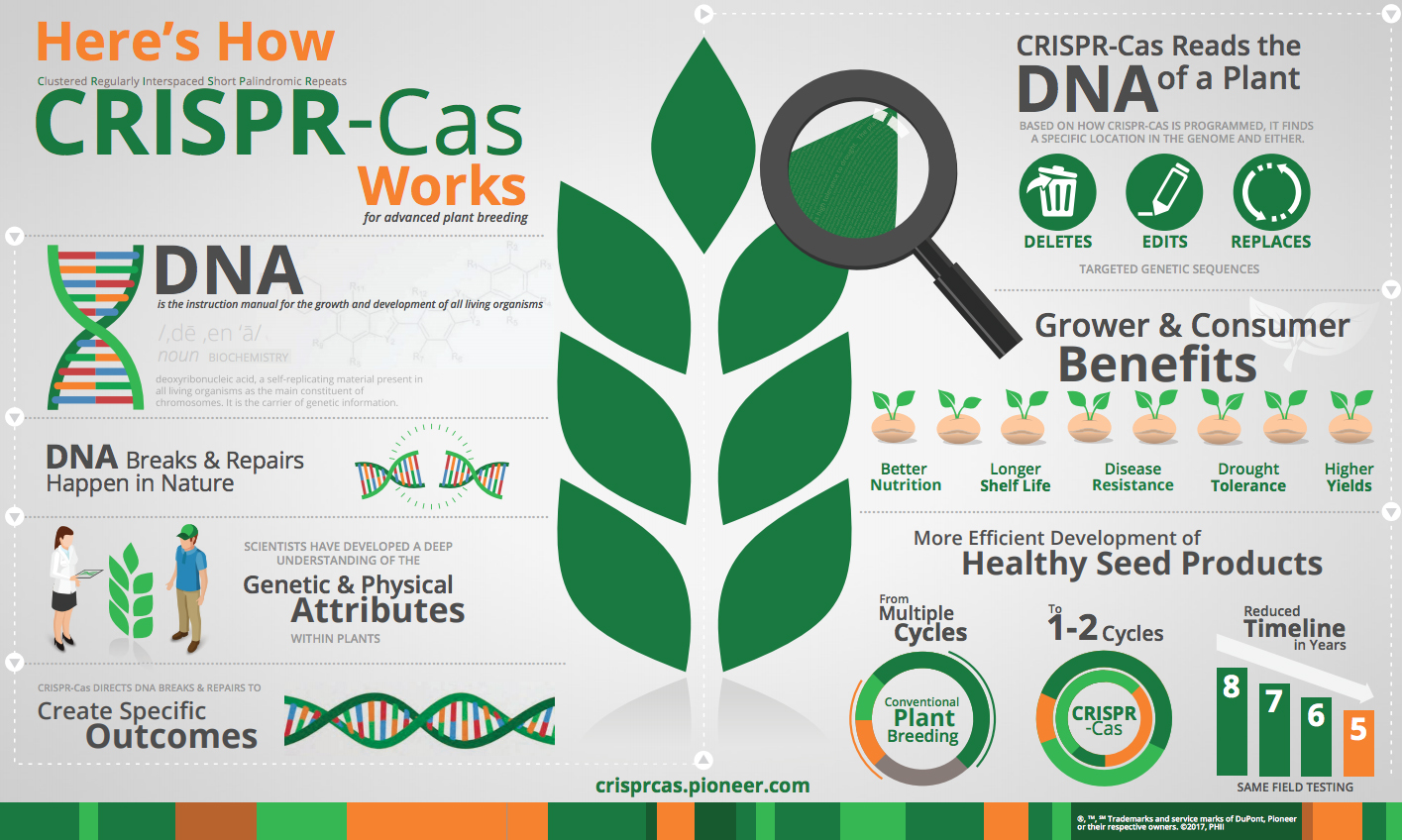 (Source: DuPont Pioneer. For more on CRISPR-Cas, go to https://crisprcas.pioneer.com
(Source: DuPont Pioneer. For more on CRISPR-Cas, go to https://crisprcas.pioneer.com
Proteins, or genes, “in the nucleus of any organism provide all the information it uses to operate and grow,” explained Jennifer Doudna, professor of molecular and cell biology and chemistry at the University of California, Berkeley, speaking at a conference on CRISPR in California. “You can think of it like a text that includes instructions and a construction manual.” Gene editing is “a way of modifying that document … you can cut and paste” the proteins in the nucleus, she said.
Speaking more technically, both CRISPR and TALEN use enzymes that sever the double-strands of an organism’s genes at targeted locations, making what is called double-strand breaks (DSBs), and often making several such cuts.

Jennifer Doudna, University of California - Berkeley
Then, DSBs are repaired by sending replacement genes to the severed site, using a sequence of genes as a template that includes the modification the breeder wants to make. Also, in both systems, other insertions or deletions of proteins are made at the break site as the broken ends are rejoined.
In the CRISPR process (also called CRISPR-Cas9), the activating enzyme is a Cas9 nuclease, and the template of replacement genes is guided to its target in a single strand of ribonucleic acid (RNA). Similarly, TALEN uses a nuclease to make double breaks, but employs a pair of double-stranded DNA binding proteins rather than a single RNA strand.
Luckily for plant and animal breeders, and perhaps all of agriculture, “there are lots of applications of gene editing,” Slutsky points out. They are used to insert genes or knock them out, tag their location on a chromosome, correct genetic defects, etc. Thus, scientists hope to use them to benefit human health, first of all, but also to edit the genes of animals, plants, bacteria, fungi, and other organisms. They want to improve livestock breeds and crop varieties, but also eliminate diseases, wipe out pathogens, rein in harmful insects, and more.
Significantly, unlike other traditional gene-editing methods, employing CRISPR or TALEN is cheap, quick and relatively easy for breeders to use. That leads Doudna to call the new gene editing processes “a democratizing tool,” because breeders in poor countries and in less-endowed labs worldwide – not just those in more lushly-funded corporate and university labs – will have broad access to gene editing.
What’s more, in October, DuPont Pioneer, a leading global crop genetics company, and the Broad Institute of MIT and Harvard, which holds the initial CRISPR patent, granted agricultural researchers at all university and other nonprofit entities free access to use the CRISPR-Cas9 patent.
With such access and potential for quick results, not surprisingly, CRISPR has swept through labs around the world. Slutsky says she travels a lot internationally to work on matters involving crop genetics, “and I probably spend 50 percent of my time in countries who are talking about (using CRISPR) … in South America, China, Japan, South Korea, Australia, Europe – countries that we would not necessarily consider biotech friendly, who ban GMO plantings. (But) they don’t want these technologies to pass them by.”
The University of California-affiliated Innovative Genomics Institute has been collecting reports of successful genetic editing by scientists worldwide. Megan Hochstrasser, IGI science communications manager, is curator for the list and she says, “the tally is over 200 right now and only includes those organisms edited using CRISPR enzymes,” not other gene editing processes.
IGI’s list includes “all organisms … vertebrates, invertebrates, plants, and microbes,” Hochstrasser reports, and “it seems that the microbes section is growing the most rapidly. Some of the microbes are pathogenic,” and she thinks that “makes sense, since scientists need to understand how pathogens function in order to combat them.”
Efforts to employ CRISPR to fight pathogens have perhaps most often been aimed at microbes that harm people: one that causes malaria, for example, and a parasite that causes Chagas disease. But researchers are also using CRISPR to build in defenses against rice blast fungus, corn smut, and the cotton bollworm, Hochstrasser noted.
Note, too, that the new processes aren’t likely to be the world’s last best answer in breeding techniques. A steam-powered car called the Stanley Rocket, for example, broke the world land speed record at 127.7 miles per hour more than a century ago (1906). Sure, that was jaw-dropping at the time, and even kind of impressive now. But, as with race cars, improvements will continue in gene-editing technique as well.
Here is one of perhaps many on the way. CRISPR actually often overperforms in the cell nucleus, making an excessive number of cuts, including some in the wrong places, scientists report. But now, University of Wisconsin-Madison researchers have found a way to improve CRISPR-Cas9 technology, making genetic revisions much more likely to be exactly as desired. Their new method uses a molecular glue, keeping the Cas9 enzyme and RNA strand together as a complete repair kit at the DNA cut.
Meanwhile, a bioengineer at the Broad Institute has been working with a family of enzymes, named Cpf1, that work much like Cas9, and scientists in other labs around the world are looking for enzymes that can be most quickly and accurately aimed at the chromosomes of living things.
Although actual commercial use of gene-edited products awaits decisions by the U.S. and foreign governments about regulating them, a lively market has already emerged to sell the enzymes, engineered strands of RNA and other molecular bits for gene editing. Online, a market exists similar to what smart-phone users find in the Google Play Store when shopping for an app.
GeneCopoeia, for example, has been marketing biotech research tools and products for nearly 20 years. The Maryland-based company has partnered with a China-based FulenGen Co. and offers an array of CRISPR and TALEN tools “to help you every step of the way in your genome editing workflow.” Meanwhile, Integrated DNA Technologies, with locations in Iowa and several sites abroad, has a similar display of products and services online.
Considering the decline in public spending on agricultural research in recent decades, will enough funding be available to sponsor cutting-edge gene-editing agricultural research? Speakers at a Farm Foundation conference on ag research and innovation last fall said they expect it will.

Gregory Graff, Colorado State University
First of all, the cost of using CRISPR and TALEN is low because of the speed of making the edits. Besides that, said Gregory Graff, an associate professor in agricultural economics at Colorado State University, with the “patent access granted to small companies and public agencies, it opens up potential for new products from small players,” and “it renders those products viable in the marketplace.”
What’s more, said Graff, who tracks research startups in the U.S., America is not capital short: “There is more money out there than there are places to invest.”
Bill Buckner, president of the Noble Research Institute in Oklahoma, agreed. “Venture capital folks don’t know how or where to invest,” leaving a lot of cash that could be available for genetic editing sorts of research. He noted, for example, a study by the Center for Rural Entrepreneurship that projects $29 trillion in wealth will be transferred between American generations from 2010 to 2040, and said “a lot of (the dollars) will be in the Midwest.”
Some of the university research will likely be conducted in collaboration with commercial firms. For example, researchers at the University of Missouri, Kansas State University and Genus plc successfully bred pigs that are not harmed by the Porcine Reproductive and Respiratory Syndrome (PRRS) virus, a disease that costs North American farmers more than $660 million annually. Click on the photo below to watch the video from University of Missouri
“Once inside the pigs, PRRS needs some help to spread; it gets that help from a protein called CD163,” said Randall Prather, distinguished professor of animal sciences in the University of Missouri's College of Agriculture, Food and Natural Resources in a December 2015 release. “We were able to breed a litter of pigs that do not produce this protein, and as a result, the virus doesn’t spread. When we exposed the pigs to PRRS, they did not get sick and continued to gain weight normally.”
Researchers working in Prather’s laboratory also created the first miniature pigs that have the alpha 1,3 galactosyltransferase gene knocked out. This groundbreaking work has the potential to prove very useful for xenotransplantation: the transfer of pig organs into humans, Prather notes on his web site.
“We also created pigs with a mutation in the gene that is responsible for causing cystic fibrosis (CF). Now there is a pig model that mimics the symptom of CF so that physicians have something to invasively experiment on and develop treatments and therapies. This is especially important since the same mutation in mice does not result in a phenotype that is similar to humans.”
More recently, scientists at the University of Missouri worked with pigs to research stem cells and made a discovery that could significantly decrease the costs associated with in vitro fertilization in humans.
Nevertheless, gene editing will have to jump a huge policy hurdle before results of such plant and animal breeding show up on farms, in fields and in food stores.
A number of scientists, consumer and food safety advocates, and others fear the results of editing genes that are all naturally within a cell's nucleus the same way they do transgenic engineering, which alters plants and animals genetically by inserting genes from unrelated organisms. They want to see the U.S. and governments worldwide lump gene editing in with transgenic genetic alterations and regulate it as just another type of genetically modified organism, or GMO. That would almost surely ensure years of testing and approval for each product, as has been done for transgenic products, dramatically running up the costs to produce gene edited products commercially.
At the California CRISPR conference, where scientists were focused on the advantages and potential of gene editing, for example, Dana Perls, a spokesperson for Friends of the Earth, declared: “Let’s actually call this (CRISPR) genetic engineering. Why not name it what it is?”



