Most Americans have never experienced a famine or even chronic food shortages. We've grown accustomed to finding at least some types of food almost everywhere we look – the grocery, the gas station, the food truck, the corner store and of course, online.
So, when scientists talk about the need to improve productivity on the farm or create new plants and animals which can resist diseases that could potentially eliminate an entire crop or species, the words often fall on deaf ears to the consuming U.S. public. Why worry?
Perhaps there is no need to panic, but there is cause for concern. Economists and analysts agree that we need to improve productivity on farms and ranches just to keep up with feeding a global population that the United Nations expects to grow from 7.6 billion to 9.8 billion by 2050.
The Global Harvest Initiative’s most recent Global Agricultural Productivity Report noted that, for the fourth straight year, agricultural productivity growth is not accelerating fast enough to sustainably feed the world in 2050.
“If agricultural productivity growth continues to stagnate, there will be significant ramifications for the economic vitality and environmental sustainability of food and agriculture systems. The availability of affordable, safe and nutritious food also will be undermined,” the report notes.
But it’s not only increased productivity that’s important. Consumers are increasingly concerned about the environmental impact of their food choices – how much water is consumed, how nutrients are utilized, and how much food is wasted. There are also concerns about nutritional benefits and the price and safety of what they eat.

From 2011-2012, a severe drought caused a food crisis in East Africa. Photo By Oxfam East Africa
Farmers are ready to meet all of these challenges, but they want access to new tools that will enable them to better cope with climate change, natural disasters and disease outbreaks. And that’s where advanced, precision breeding shows so much potential.
“Different forms of breeding can address these challenges,” emphasized Ian Jepson, Syngenta’s Head of Trait Research and Development Biology, during a recent interview. He says the key challenges are the biotic stressors - the weeds, the insects, the fungal diseases and other pathogens, like nematodes, bacteria and viruses which all can significantly impact yields. However, the biggest losses are through abiotic stress, like heat, drought and cold.
“It’s been estimated that $200 billion in losses a year are due to those biotic stressors,” Jepson points out. “By 2050 we’ve got to increase productivity not only by protecting the crops from the bugs, but we’ve got to address fundamental yield and biotic stressors.
“Chemistry and biologicals do a great job on the biotic stressors. We have good chemical controls for weeds,” Jepson adds. “We don’t have good chemical controls for insects and fungal diseases and we’re struggling to get new products to keep up with resistance pressures. So, on the biotic stressors, we need to supplement our chemistry and our biologicals with advanced breeding."
In order to increase a crop’s yield, Jepson says “we can’t keep throwing more nitrogen on it, because we’ve got runoff issues. We’ve got to work on the inherent productivity of crops. And we know that potential is there.”

Ian Jepson, Syngenta’s Head of Trait Research and Development Biology. Photo courtesy of Syngenta.
“If you take a crop like sugar cane and consider its photosynthetic capacity, you’ll find that its ability to use the sun’s energy and water to make sugars is way more efficient than most other crops. So, if we get the poor crops up to the level of the good crops, we can increase yields by 40 to 50 percent,” he explained.
“But we’ve got to figure out how to do that. It’s advanced breeding techniques that are going to make those breakthroughs.”
Jepson says there are a number of different efforts underway that can boost plant yields that now use only about 1 percent of the sunlight they receive in photosynthesis. For example, Realizing Increased Photosynthetic Efficiency (RIPE) is an international research project, headquartered at the University of Illinois, that is engineering plants to photosynthesize more efficiently to sustainably increase crop yields.
Formed in 2012, RIPE was originally funded by a five-year, $25-million grant from the Bill and Melinda Gates Foundation. In 2017, the project received a $45 million, five-year reinvestment to continue its work from the Gates Foundation, the Foundation for Food and Agriculture Research, and the U.K. Department for International Development.
“Photosynthesis is the process from which ultimately all our food and a lot of our fiber and many of our fuels actually come from,” said Stephen Long, a professor of plant biology and crop sciences at the University of Illinois in an interview with Illinois Public Media. “And the process really isn’t that efficient.”
Researchers found that by boosting levels of three proteins in tobacco plants, the crop grew 14 percent to 20 percent larger, according to a study published in Science in 2016. And they are confident that this process can be transferred to other crops, such as corn and soybeans which are widely planted in the U.S., or cowpeas, planted by small stakeholder farmers in Africa.
Meanwhile, researchers at the Donald Danforth Plant Science Center in Creve Coeur, Missouri, aim to identify new genes and pathways that contribute to photosynthesis and enhanced water-use efficiency - building on earlier research using the model grass, green foxtail (Setaria viridis).
“Understanding the network of genes involved in photosynthesis and drought tolerance will provide targets for plant breeders and genetic engineers to redesign sorghum specifically as a high value bioenergy feedstock to be grown on marginal soils and thus not compete with food crops,” said lead principal investigator, Thomas Brutnell, director of the Enterprise Rent-A-Car Institute for Renewable Fuels at the Danforth Center.
 Ultimately, they hope to deliver stress-tolerant sorghum lines, addressing the Department of Energy's (DOE's) mission in the generation of renewable energy resources. The development of a low input, environmentally safe and highly productive sorghum germplasm will help establish a lignocellulosic energy economy that can provide jobs to rural communities, ensure energy security and benefit the environment, the Center noted after receiving a five-year, $16 million grant from DOE in October 2017.
Ultimately, they hope to deliver stress-tolerant sorghum lines, addressing the Department of Energy's (DOE's) mission in the generation of renewable energy resources. The development of a low input, environmentally safe and highly productive sorghum germplasm will help establish a lignocellulosic energy economy that can provide jobs to rural communities, ensure energy security and benefit the environment, the Center noted after receiving a five-year, $16 million grant from DOE in October 2017.
More recently, Andrea Eveland, an assistant member at the Danforth Center, and her team identified a genetic mechanism that controls developmental traits related to enhanced grain production in cereals. The work was also performed on Setaria viridis, which is related to economically important cereal crops and bioenergy feed stocks such as maize, sorghum, switchgrass and sugarcane.
“The genetics and genomics tools that are emerging for Setaria enable more rapid dissection of molecular pathways such as this one, and allow us to manipulate them directly in a system that is closely related to the food crops we aim to improve,” said Eveland. “It means we are just that much closer to designing and deploying optimal architectures for cereal crops. The prospect of leveraging these findings for improvement of related grasses that are also orphan crop species, such as pearl and foxtail millets, is especially exciting.”
Syngenta has been making advances in breeding, too. Their scientists solved the mystery behind an abnormal corn line responsible for revolutionizing corn breeding. The line produces haploid plants that contain just half the DNA of normal corn and was first discovered in 1959 by University of Missouri Professor Edward Coe.
“We (the seed industry) make millions of plants using this particular mutant line,” Jepson explained. “But we had no clue how it worked – until recently.”
They found their answer in 2013 and followed up with gene editing to verify the discovery in 2015. As a result, Syngenta hopes to make existing haploid-induction systems more efficient and potentially make breakthroughs in other crops.
All in all, researchers have made tremendous advances in plant breeding using a variety of different tools and relying on big advances in computational biology and computer storage that allow analysis of petabytes of data. But there’s still a long way to go. And for some growers, help can’t come fast enough. In some cases, entire farms, businesses and food supplies are being wiped out.

Cassava Brown Streak disease is devastating a staple crop in parts of Africa.
For example, Brown Streak Disease is devastating cassava plants in many African countries, especially in East Africa, where the root vegetable is a staple food for millions. In some cases, the disease, which has been dubbed the "Ebola of plants,” exposes farmers to 100 per cent loss, notes Biosciences for Farming in Africa.
Nigel Taylor, with the Danforth Center, is working with scientists in Uganda and Kenya to see if a relatively new-gene editing technology – CRISPR (stands for Clustered Regularly Interspaced Short Palindromic Repeats) - can be used to speed up the time it takes to grow cassava plants that are more resistant to the disease than conventional varieties.
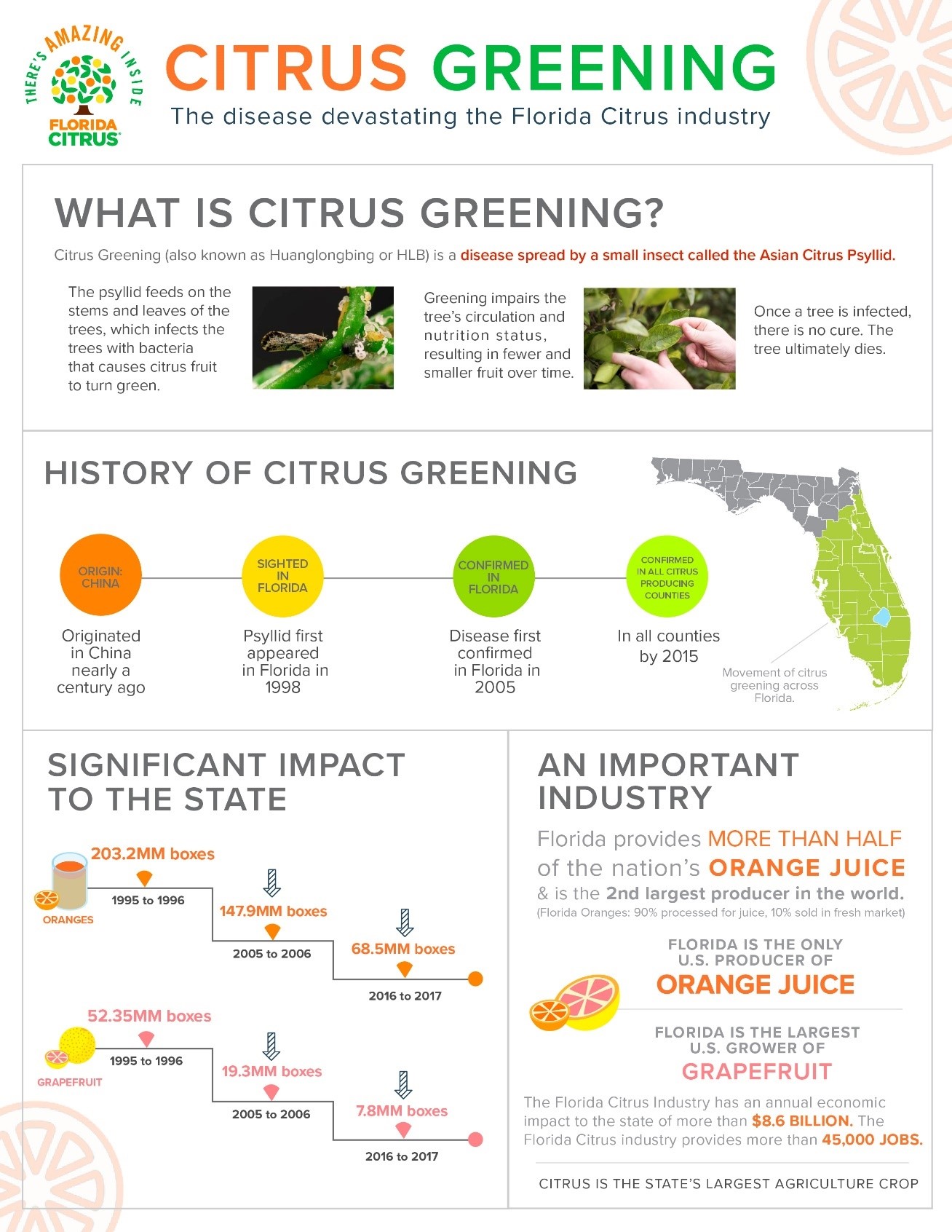
In the United States, researchers are trying to find a cure for another particularly vexing problem - citrus greening disease, which was first confirmed in Florida in 2005. The Asian citrus psyllid (ACP) carries a bacterium infesting trees with huanglongbing (HLB or citrus greening). Infected trees produce fruits that are green, misshapen and bitter. Most infected trees die within a few years.
USDA reported in 2017 that HLB is currently the most devastating citrus disease worldwide and has now affected all of Florida's citrus-producing areas leading to a 75 percent decline in the state's $9 billion citrus industry. Fifteen U.S. states or territories are under full or partial quarantine due to the presence of ACP.

Citrus tree leaves infected with citrus greening. Source: USDA - APHIS
Although there is no cure for the disease, growers have implemented several short-term solutions, including enhanced nutritional supplements, reflective mulch, bactericides and heat treatment to try to maintain fruit production.
Longer term, researchers are looking at both traditional cross-breeding and new processes like gene editing and genetic modification to explore how to develop a disease-resistant breed of citrus tree which is not susceptible to greening and will not become diseased.
USDA’s National Institute of Food and Agriculture (NIFA) recently announced new funding to combat the disease.
“The need to advance research and extension to develop management strategies for huanglongbing has reached a critical juncture,” said NIFA Director Sonny Ramaswamy. “Severe damage to the Florida citrus crop from 2017 hurricanes further exacerbates the pressure on the industry and the need for new strategies to address the disease.”
Florida’s citrus industry has lost nearly half of its $1.5 billion on-tree fruit value in just 10 years due to citrus greening.
Harold Browning, the chief operating officer for the Florida-based Citrus Research and Development Foundation, says researchers in Florida, Texas and California continue to produce new citrus strains along with pursuing HLB resistance.
Yet, all types of citrus remain vulnerable to HLB to varying degrees, Browning says, and scientists haven’t yet developed any commercial citrus varieties with strong resistance to the disease. “We really don’t have a variety that is equivalent to a boat sitting on the pond with no holes in it,” he says.
https://www.floridacitrus.org/newsroom/citrus-411/citrus-greening/citrus-greening-infographic/
Pete Spyke, a veteran citrus grower who runs Arapaho Citrus Management in Fort Pierce, Florida, says there are no commercial citrus varieties that have been gene-edited or genetically-engineered because the work is still in research phases.
Researchers at USDA, the University of Florida, University of California, Texas A & M and other universities are working to find a source of tolerance, resistance or immunity, Spyke says.

Peter Spyke, Arapaho Citrus Management
“Once they do, they will have to go into every single variety and perform that edit. And then the tissue of that new edited variety has to be grown out and propagated material generated so that we can begin to propagate new commercial citrus trees.
“So, if someone arrived at my doorstep today with, for example, a perfectly-edited variety of navel orange, it would still probably be 10 years before there was an industry based on that new variety,” he adds. “Every year we go, it’s another 10 years from today.”
The need for speed
For growers like Spyke, these advancements in genomics and precision plant breeding can’t come fast enough.
But for the science to really take off, some big hurdles need to be overcome. Researchers are hungry for a federal and international regulatory system that clarifies how different plant varieties should be regulated. Plus, they’d like to see broad acceptance of these new breeding techniques by all parts of the food supply chain, including consumers.

NIFA Director Sonny Ramaswamy
“The EPA, USDA and FDA have to come up with an actual definitive regulatory framework,” says NIFA’s Ramaswamy. “Currently they don’t have one in regards to gene editing.”
For now, USDA has concluded that the new plants are not “regulated articles.” But not everyone sees it that way.
There’s already been some push-back on these new tools, driven primarily by people and organizations who either don’t understand how the technology works or who aren’t comfortable with anything they view as “messing with Mother Nature.”
What most people forget is that Mother Nature has been changing plants for centuries, and that these new precision breeding tools can make the changes faster and more precisely.
“DNA is inherently stable, but breaks from time to time. And when it breaks, that break can be caused by UV light or chemicals or heat or mechanical damage,” explains Syngenta’s Jepson. “And when it breaks, it sticks itself back together. When it does that, it has a chance of making a mistake and that creates variation. That’s how editing works. But this is a random process and not very efficient.”
Sweet corn is one example, he explains. “While regular corn in the field, you wouldn’t want to eat it or put it on the grill. It’s starchy. Sweet corn comes from one of the starch genes that has been broken and repaired and made a mistake. Instead of producing starch, it stays sweet and sugary. And that’s how sweet corn was derived … where random mutations happened in nature and then were selected by plant breeders.”
Starting in the 1920’s, scientists discovered how to induce variation through mutagenesis breeding, sometimes called radiation breeding.
“They (researchers) would use a range of different techniques like x-rays or chemicals or they put plants through tissue culture and it would just increase the rate of mutation,” Jepson explains. “Using those processes, they would generate populations of thousands of plants and they would put them in the field. And most of them were mutations that were not beneficial. They would just disrupt pathways of interest so they would throw those ones away.”
“The technique is still used today, sometimes called tilling,” he adds. “It’s a bit more sophisticated, but it’s still around.”
Thousands of crop varieties have been developed using these mutagenesis approaches, including sweet potatoes, durum wheat for pasta, and the Ruby Red Grapefruit. Other varieties can be found on the joint Food and Agriculture Organization/International Atomic Energy Agency Mutant Variety Database.
Ironically, grocers and food companies have been selling crops produced through mutation breeding for decades without a label or any apparent consumer backlash about their genetic changes from chemicals or radiation. These varieties can even be labeled organic if they are grown according to other organic production requirements.
Yet, new precision breeding tools are creating such a buzz that some activists suggest techniques like CRISPR Cas9 - which involves “cutting and pasting'' DNA within a plant at specific sequences – should be regulated the same as genetically-modified organisms (GMOs), which are created by the insertion of genetic material from a different species.
There is no science-based risk associated with either form of breeding, but GMOs have gotten a bad rap from several environmental groups which have pressured food companies to avoid them in food products.
Even the Non-GMO project, which attempts to verify and label food and beverage products that do not contain GMOs, wants to exclude gene-edited foods from consideration – even if they are not technically GMOs.
As a result, farmers and plant breeders are worried that much-needed research – aimed at solving some of the most pressing plant diseases - is at risk of being stymied in the commercial marketplace over unfounded fears about GMOs. Gene-editing could be one way around that barrier.
“I’m comfortable with GMOs, but big juice companies are skittish about utilizing them for brands like Tropicana or Minute-Maid orange juice,” Spyke says. “So, at this point, the assumption is that for the main citrus industry (oranges for juice), the GMO route – where we introduce a foreign gene - is not the preferred solution.
“Using CRISPR for gene editing is the most hopeful at this point because it doesn’t change the fundamental citrus genome,” Spyke adds.
Some firms are already marketing gene-edited plants as non-GMO or working to do so in the near future. And because the production and regulatory costs associated with gene editing are so much lower, a lot of smaller technology companies are jumping into the action alongside much bigger seed companies.
For example, San Diego-based Cibus developed sulfonylurea-tolerant canola using non-transgenic breeding technologies. Jim Radtke, Cibus’ senior vice president for product development, says, the company is “making changes in plants without incorporating foreign DNA and thus the plants are non-GM,” using a patented gene-editing tool called the rapid trait development system (RTDS). And companies like Cargill are paying a premium for the canola to make non-GMO oil.
Cibus expects to introduce non-transgenic glyphosate-tolerant flax in 2019, late blight resistant potato in 2020 and a herbicide tolerant rice after that.

Jim Radtke, Cibus’ senior vice president, product development. Photo: Cibus
Calyxt, is using a gene editing technique called TALEN, which is similar but not identical to the CRISPR Cas-9 gene-editing tool, to develop new crops. The Minnesota-based firm, which bills itself as a consumer-centric, food- and agriculture-focused company, is preparing for the commercial launch of its first product, high oleic soybeans, in 2018. Also in the Calyxt pipeline: a potato variety that doesn’t bruise and another that survives better in cold storage, high-fiber wheat, low-gluten wheat, herbicide tolerant wheat, and lower saturated fat canola.
Building off technology developed at the University of Missouri, Yield10 Bioscience Inc. developed a gene-edited Camelina sativa plant line using CRISPR technology for increased oil content. The firm says it is “focused on translating initial encouraging yield improvement results in Camelina to canola, soybean, rice and corn.”
Syngenta, which has its U.S. headquarters in Greensboro, N.C., and its Advanced Crop Lab in the state's Research Triangle Park, recently obtained a non-exclusive license from the Broad Institute of MIT and Harvard to use CRISPR-Cas9 technology for agricultural applications. Syngenta said it will use CRISPR-Cas9 in various crops, including corn, soy, wheat, tomato, rice and sunflower.
Berkeley-based Caribou Biosciences, in partnership with DuPont Pioneer, is using CRISPR Cas-9 to produce a waxy corn. This next generation of elite waxy corn hybrids is expected to be available to U.S. growers within the next few years, pending field trials and regulatory reviews. DuPont Pioneer says it is establishing a CRISPR-Cas enabled advanced breeding platform to develop seed products for greater environmental resiliency with characteristics like disease resistance and drought tolerance, in addition to advancing the development of improved hybrid systems.
Monsanto has licensed two different CRISPR versions, CRISPR-Cas and CRISPR-Cpf1- which the firm describes as having the potential “to be a simpler and more precise tool" for making targeted improvements in a cell's DNA - as well as the Exzact technology and another gene-editing platform developed by TargetGene Biotechnologies Ltd. The company is focusing on potential improvements in corn, soybeans, cotton and vegetables in ways that will make farmers more profitable.
Germany-based Bayer, which is in the process of acquiring Monsanto, has its own joint venture centered on CRISPR gene editing and is expected to continue building on Monsanto’s existing portfolio of tools.
Plenty of foundations and university researchers are also using new gene-editing techniques to improve vegetable crops, including tomatoes resistant to powdery mildew and virus-resistant cucumbers.
Scientists at the Noble Institute Research are using gene editing technology to improve the cover crop hairy vetch. Noble researchers are looking to improve the germination of seeds to make this legume more functional as a cover crop.
In 2016, Penn State University pathologist Yinong Yang used CRISPR-Cas9 to develop a button mushroom that resists browning, may have a longer shelf life and be better for automated mechanical harvesting. In approving the new mushroom, USDA wrote that because it “does not contain any introduced genetic material” it isn't subject to the agency's GMO regulations.
Regulatory uncertainty
But will new plant varieties produced using various forms of precision plant breeding continue to be regulated this way? That’s potentially a multimillion dollar question. Farmers, researchers and investors would like some type of regulatory certainty in order to fully explore the potential to boost yields, protect plants from disease and provide added nutritional benefits.
“The challenge is this: You may get the regulatory authorities in the United States for some of those modified oils that don’t need any regulatory permits to produce in the United States. But Europe and many other countries have not yet decided what is going to be required, if anything,” noted Jepson. “That’s the very limiting step here. How soon will the regulatory frame become established enough?"
Michal Bobek, advocate general of the European Court of Justice, recently delivered some relatively good news on that front. On Jan. 18, he ruled that new gene-editing technologies should be largely exempted from EU laws on GM food, although individual EU member states can regulate them if they choose.
That opinion drew a swift rebuke from Friends of the Earth Europe.
“Farmers and consumers across the EU expect that any new approach to producing food and crops should be fully tested to make sure they are safe for the public and the environment,” said Mute Schimpf, food and farming campaigner at Friends of the Earth Europe. “They will be counting on the European Court of Justice (ECJ) to not uphold (Bobek's) opinion, and instead makes sure that all new genetically modified foods and crops are properly regulated."
The ECJ is expected to make its final ruling in the coming months, taking into account the opinion. The European Commission is waiting for clarification from the courts before deciding whether new legislation – or an update of existing laws – could be needed for the new technology.
In Australia, regulators are proposing changes more in line with the current U.S. view.
Australia's gene technology regulator Raj Bhula recently proposed reducing regulations around CRISPR and other gene editing techniques, noting that they would not be considered "genetic modification".
"With gene editing you don't always have to use genetic material from another organism, it is just editing the [existing] material within the organism," Bhula told ABC Rural. "If there is no risk case to be made when using these new technologies, in terms of impact on human health and safety for the environment, then there is a case for deregulation.”
For more news, go to: www.Agri-Pulse.com


